The Promise and Challenges of CGRP as a Migraine Biomarker
Posted on May 03 2024,
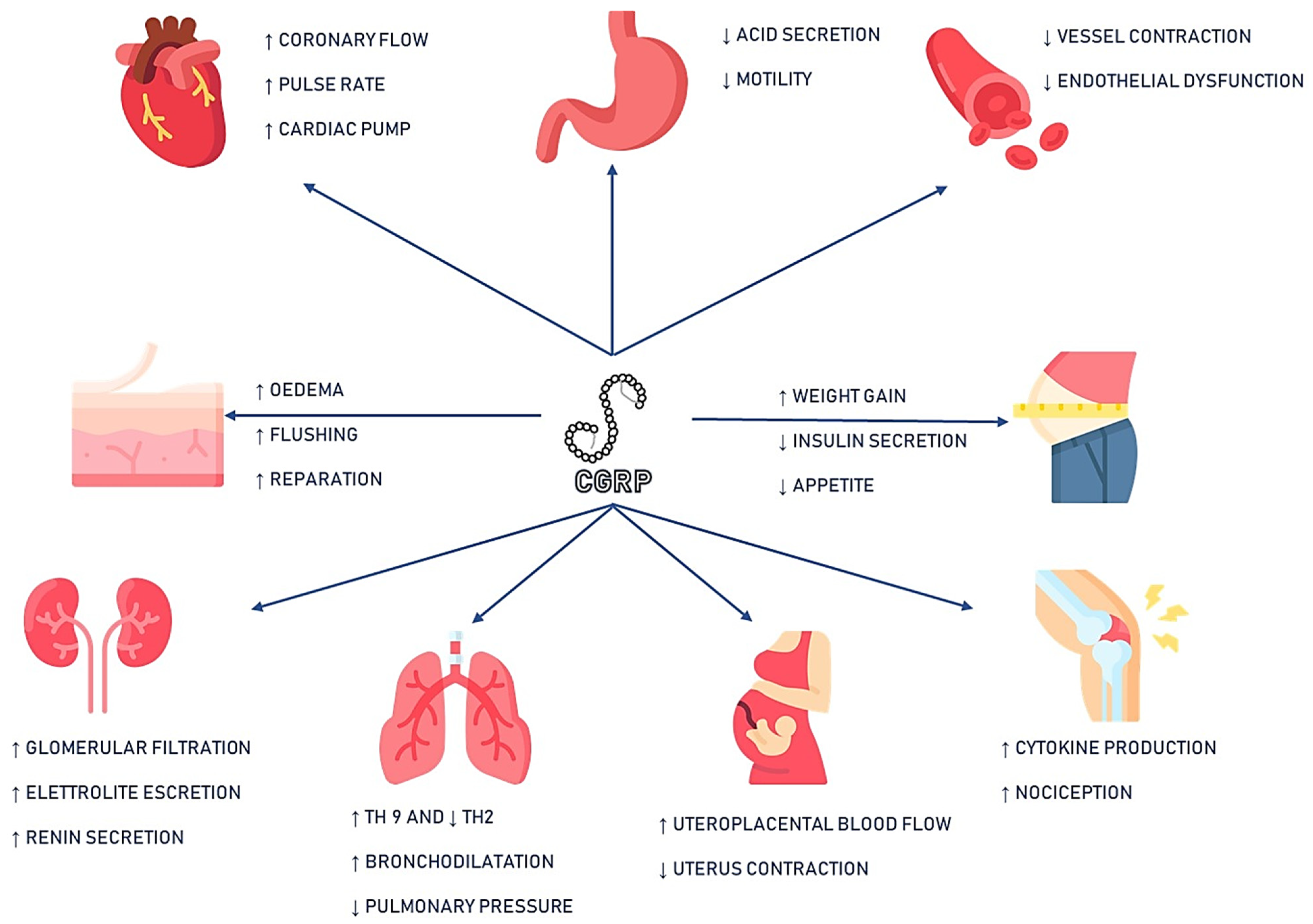
While diagnosis and treatment monitoring of migraine disease largely rely on patient-reported symptoms, the discovery of calcitonin gene-related peptide (CGRP) as a potential biomarker has sparked hope for a more objective approach to managing this complex condition.
What is CGRP?
CGRP is a neuropeptide that has been implicated in the pathophysiology of migraine. Studies have shown that CGRP levels are elevated in some migraine patients, particularly during attacks, and that targeting CGRP with medications can provide relief for many people. However, the studies are also conflicting with some finding no difference in CGRP levels between migraine patients and the general population.
Challenges in Validating CGRP as a Migraine Biomarker
Despite the promise of CGRP as a biomarker, validating its reliability has been challenging. A recent review of the existing literature revealed significant variability across studies in terms of study design, CGRP determination methods, results, and conclusions. This lack of consistency suggests that methodological differences may be contributing to the discrepancies observed in the field.
Those include:
- ELISA Kit Variability
The specific ELISA kit used for CGRP measurement has been shown to impact results. Some kits have poor performance, which means we need validation of these kits before use in research or clinical settings.
- Alpha-CGRP and Beta-CGRP Isoforms
Alpha-CGRP and beta-CGRP, two isoforms of the peptide, have been found to have different median levels in tested samples. Therefore, these isoforms need to be measured separately, as they may behave differently in the context of migraine pathophysiology.
- CGRP Stability in Serum
CGRP remains stable for 24 hours when stored at 4°C after clotting and centrifugation. However, long-term storage at -80°C for over 6 months can lead to a decrease in CGRP levels, which is important for ensuring the integrity of samples in future studies.
- Demographic Factors
Alpha-CGRP levels were higher in males, while beta-CGRP positively correlated with age. These findings emphasize the need to consider demographic factors when interpreting CGRP results and making comparisons between groups.
Establishing Normal Data Ranges
Determining appropriate reference ranges for biomarkers is a time-consuming and complex process. Laboratories must analyze samples from a large, diverse population of healthy individuals to establish a baseline and account for potential variations due to factors like age, sex, and ethnicity. In the case of CGRP, more work is needed to establish reference values that can be reliably used in clinical settings.
Sample Source Considerations
The source of the sample can significantly impact the observed CGRP levels and the interpretation of results. CGRP has been measured in various biological fluids, including blood, cerebrospinal fluid, saliva, and tears. Further research is needed to determine the most appropriate sample source for CGRP measurements in the context of migraine.
Non-Specificity of CGRP Release
CGRP is not exclusively released from the trigeminal system but can also be secreted from other sources in the body, such as the enteric nervous system and the cardiovascular system. This non-specificity of CGRP release may contribute to the variability observed in CGRP measurements and complicate the interpretation of results in the context of migraine.
Final Thoughts
While CGRP holds great promise as a migraine biomarker, the road to its validation and clinical implementation still has many obstacles. By standardizing methods, carefully validating assays, accounting for potential confounding factors, and considering the impact of sample source and collection procedures, scientists can work towards resolving the inconsistencies in the CGRP migraine literature and ultimately allow CGRP to finally be used as a valid migraine biomarker.
Tue, Mar 11, 25
Biofeedback Types for Migraine Treatment
Biofeedback is a non-invasive, non-pharmacological approach that enables individuals to gain control over physiological processes linked to migraine, such as muscle tension, blood flow, and brain activity. Using monitoring equipment,...
Read MoreMon, Mar 10, 25
Chronic Pain Conditions Associated with Migraine
Chronic Pain Conditions Associated with Migraine Condition Description Association with Migraine References Irritable Bowel Syndrome (IBS) A functional gastrointestinal disorder characterized by abdominal pain and altered bowel habits. Higher prevalence...
Read More


